
For a few amino acids, all atoms are indicated in balls and sticks to highlight intra and inter atomic interactions. The protein chains are shown in ribbons of different colors. The formation and the stability of their native structure involve intramolecular atomic interactions (interaction within one chain) to acquire their fold and intermolecular atomic interactions (interaction between chains) to acquire their quaternary structure. Protein oligomer.Oligomeric proteins need to assemble several copies of their chain to perform their biological function. The formation and the stability of their native 3D structure involve only intramolecular atomic interactions (interaction within a chain). Protein monomer.Monomeric proteins perform their biological function with a single chain. Here the protein chain is considered as the molecule. These are intermolecular (between two molecules) amino acid interactions ( Fig. Chain association involves the formation of interactions/bonds between atoms of the amino acids provided by at least two individual chains. These are intramolecular (within a single molecule) amino acid interactions ( Fig. įolding involves the formation of interactions/bonds between atoms of the amino acids of a single chain. Icosahedral is not a cubic point group symmetry but has been conflated to the cubic point group symmetry in chemistry. Ferritin is given as an example (PDB 1K4R). I, icosahedral with twelve 5-fold axes, twenty 3-fold axes and thirty 2-fold axes. One ferritin is given as an example (PDB 1LB3).

O, octoahedral, octahedron or hexahedron with six 4-fold axes, eight 3-fold axes and twelve 2-fold axes. Ferritin is given as an example (PDB 1DPS). T, tetrahedral with four 3-fold axes and six 2-fold axes. One ferritin is given as an example for a D2 symmetry (PDB 2RBD).
#PROTEIN OLIGOMERS HYDROPHOBIC AMINO ACIDS PLUS#
Dn, dihedral with n rotational axes plus 2-fold perpendicular axes. One ferritin is given as an example for a C3 symmetry (PDB 2F7N). C.Protein oligomers belong to three different point group symmetries. B.Distribution of taxa according to point group symmetries. A.Distribution of taxa according to quaternary structures. Protein oligomer features.The data in A and B are obtained by screening the PDB. In forming a protein oligomer, subunit association has to be considered in addition to folding. Besides dimers, there exists a large variety of assemblies in terms of quaternary structures and point group symmetries ( Fig. A taxon is a set of living organisms grouped because of some shared characteristics. According to the Protein Database (PDB) where all known 3D structures of proteins are stored, the most observed quaternary structure in all taxa is the dimer ( Fig.
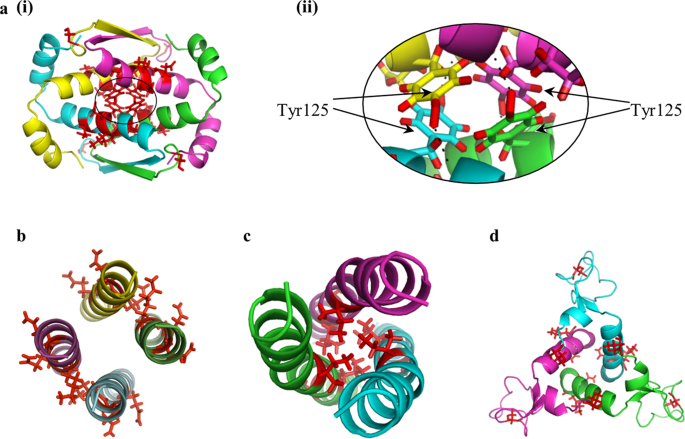
The number of associated chains defines the quaternary structure of the oligomer, or its stoichiometry. Homo-oligomers have chains with identical sequences and hetero-oligomers have chains with different sequences.
The vast majority of proteins are oligomers which function only after the association of several copies of their chains. The set of reactions leading to the native structure is the folding of the protein. The functional shape is the native structure of the protein. Based on the sequence and the environment, the protein acquires a tridimensional shape called tertiary structure (3D-structure), conformation or fold, suitable for its biological function. Proteins are biological entities made of a chain of amino acids bound to one another in a specific order, called the primary structure or the amino acid sequence of the protein.


 0 kommentar(er)
0 kommentar(er)
